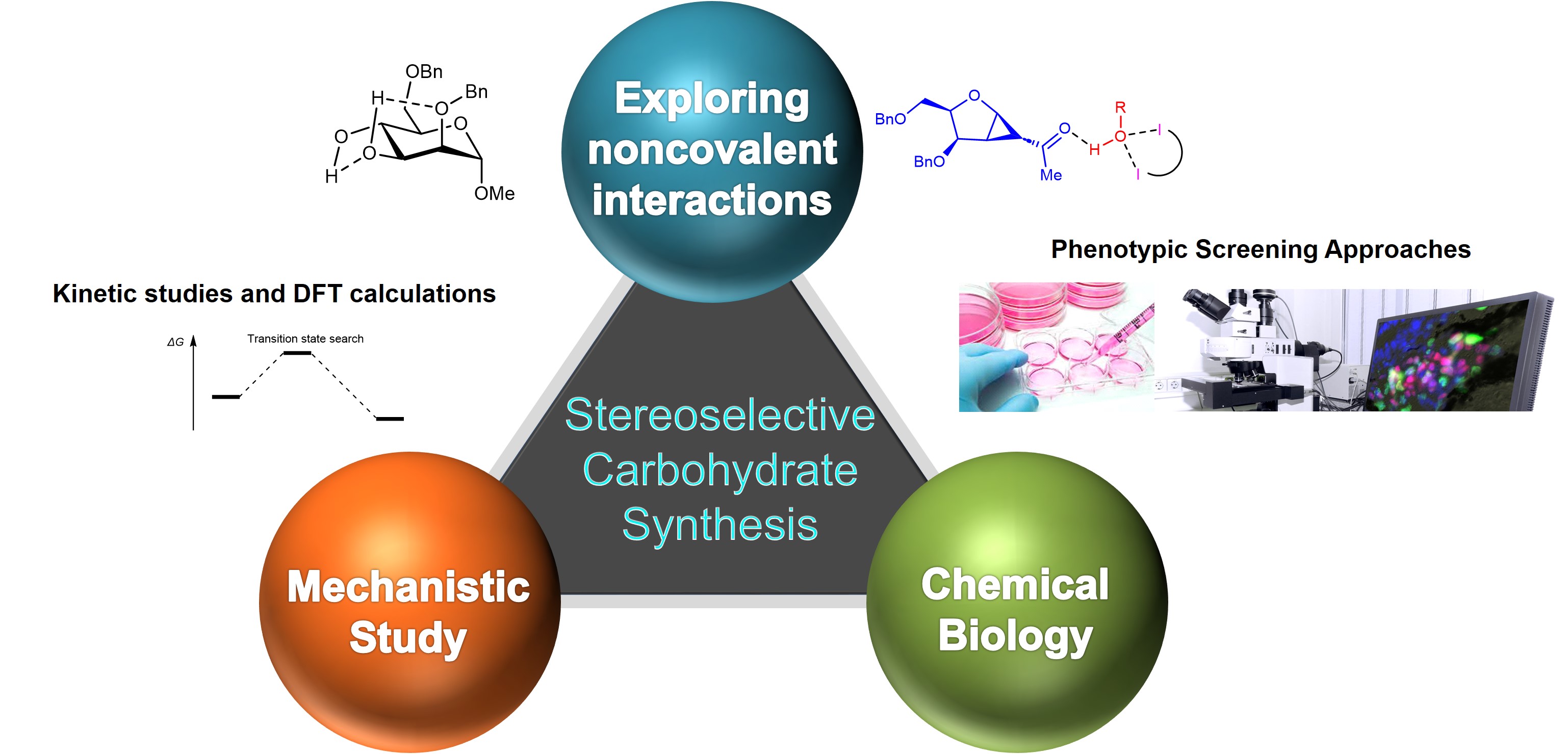
The research work in the Loh research group harnesses nature inspired noncovalent interactions (NCIs) and emerging catalytic concepts to advance carbohydrate chemistry. We use a mechanistic driven approach to understand NCI intricacies of catalytic glycosylation/glycofunctionalization pathways, in particular by kinetic experiments and DFT computations. We have a long-term interest in unraveling synthetic capabilities of the broad palette of NCIs including and beyond the ubiquitous hydrogen bond.
Our research programme is highly interdisciplinary, spanning a range of chemistry fields including synthesis, methodology development, catalysis, supramolecular chemistry, computational chemistry and forward based chemical genetics (phenotypic cell based assays to discover new biological activity). This modern chemical approach opens up a plethora of opportunities to answer innovative research questions at the interface of major chemical research themes.
Our research direction is currently divided into the following 3 strategic lines:
a) Exploitation of noncovalent interactions beyond hydrogen bonding in stereoselective carbohydrate synthesis
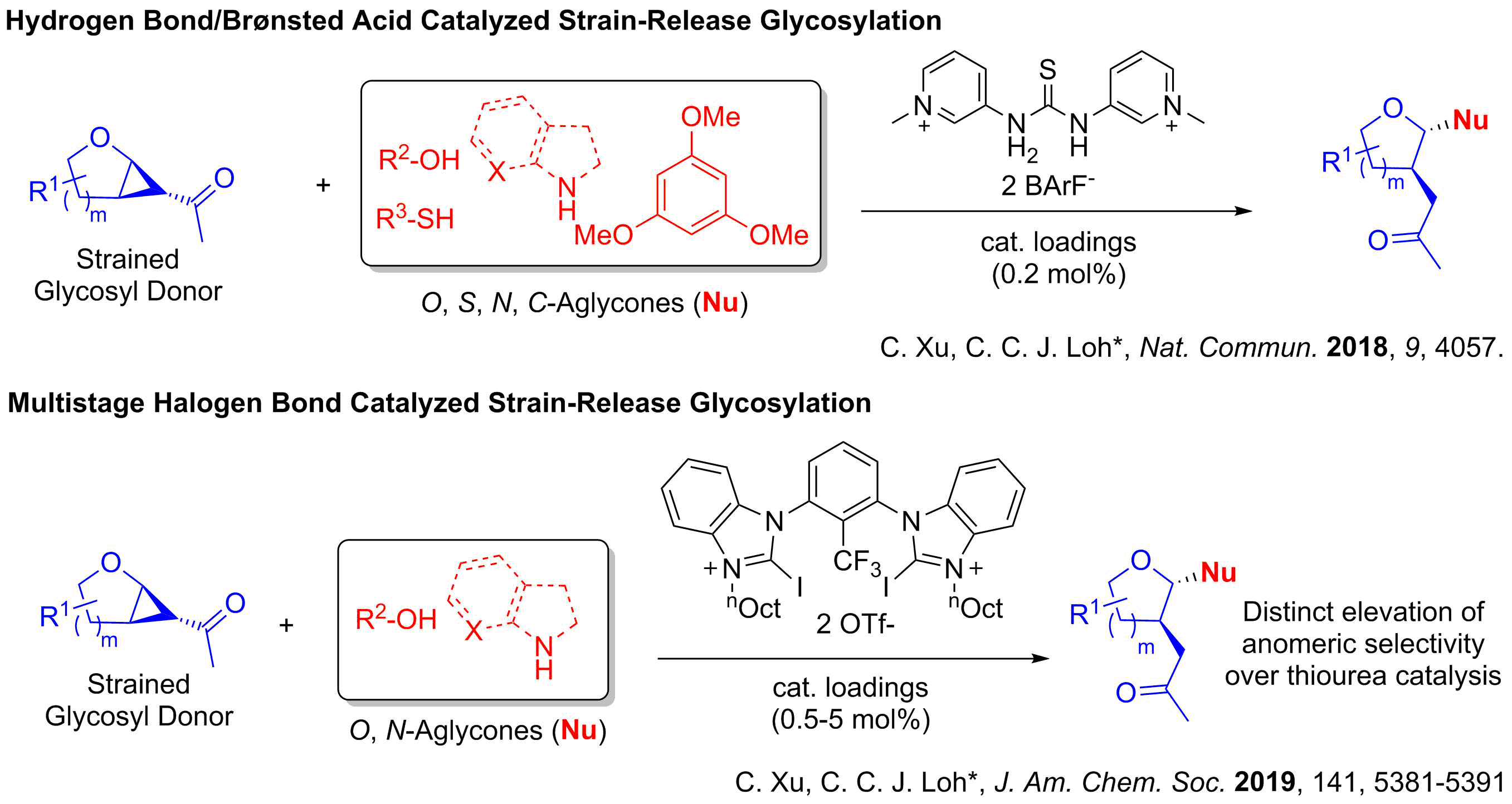
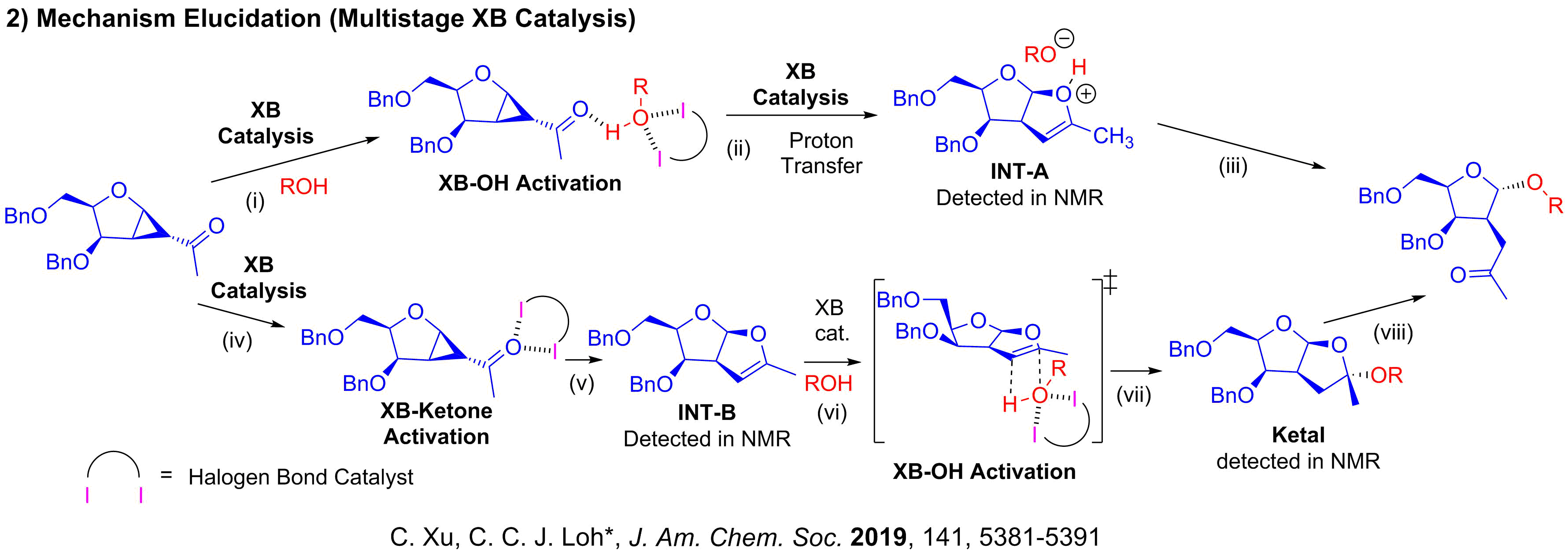
Inspired by how enzymes such as glycosyl-transferases perform precision stereocontrolled chemistry simply by the use of hydrogen bonds, our pioneering work involved investigating how noncovalent concepts such as hydrogen bonding (HB) and halogen bonding (XB) could modulate glycosylations. Currently, our research group has successfully published our seminal reports demonstrating the power of HB and the XB catalysis to construct glycosidic bonds via versatile strain-release glycosylations. We also demonstrate that sigma hole based XB catalysis can offer unique opportunities, such as improved versatility in difficult carbohydrate substrates, in 2-deoxyglycosylations unachievable via alternative methods.
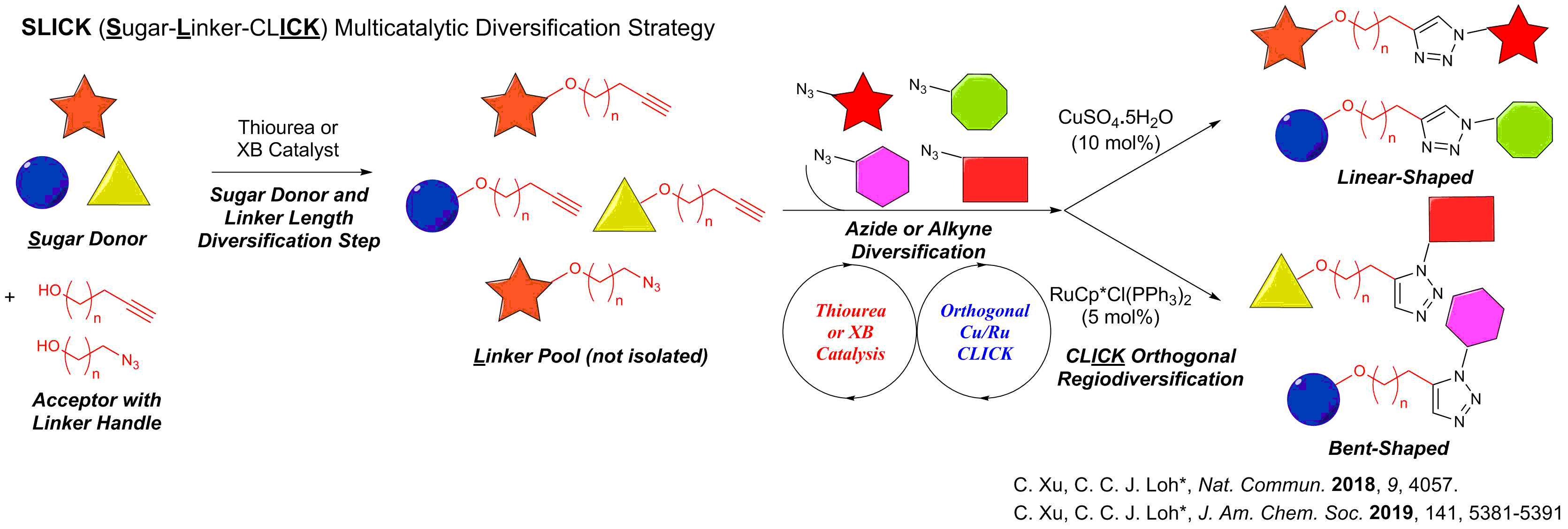
We have also developed a generally applicable multicatalysis diversification concept termed SLICK (Sugar-Linker-CLICK), where a one-pot sequential catalytic operation provides rapid diversification into structurally diverse glycosides.
b) Mechanistic Investigations via kinetics studies and DFT computations to support experiments
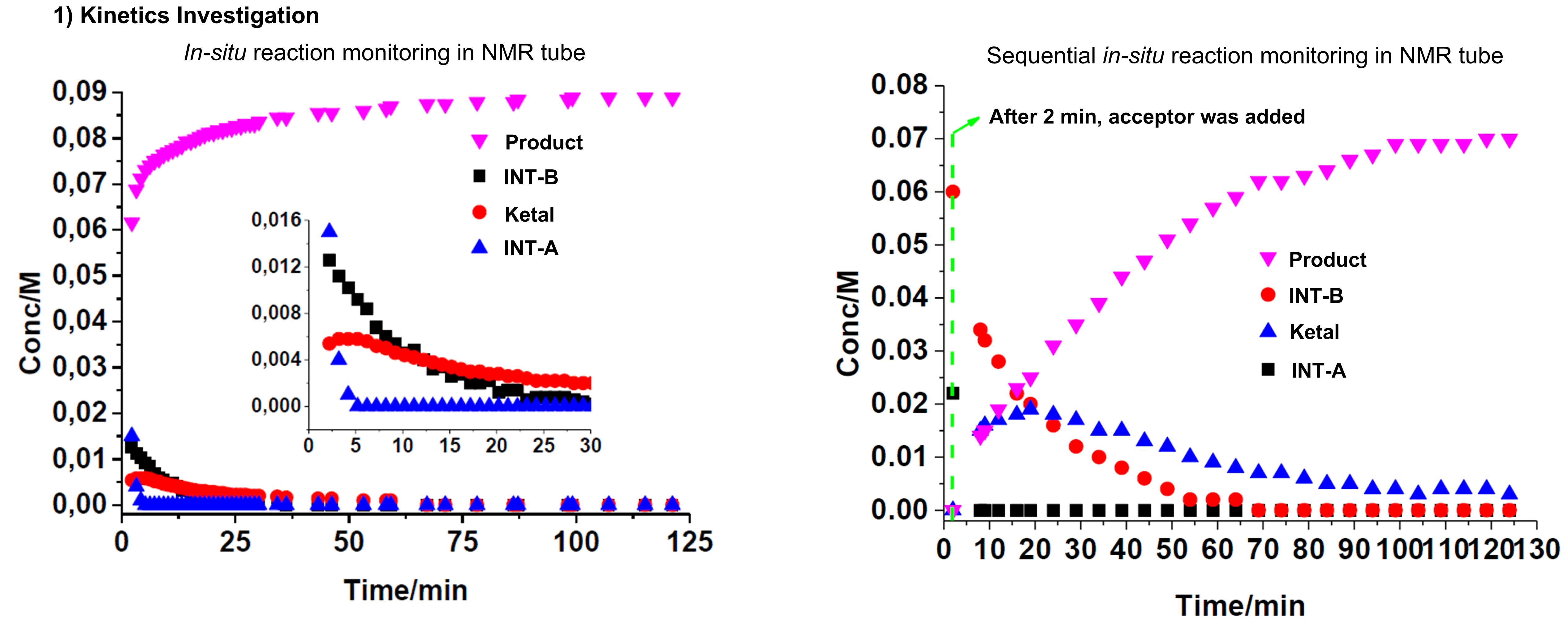
We are interested to advance carbohydrate chemistry by unraveling the mechanistic intricacies via rigorous in-situ NMR and IR-monitoring and kinetics analysis. This mechanistic driven approach is a powerful tool that enables us to dissect the mechanistic underpinnings of glycosylation pathways involving NCIs. We have currently also established an in-house platform for modern DFT calculations, and we harness a range of frontier computational tools to provide theoretical support for mechanistic questions that involves NCIs. We employ state-of-the-art DFT functionals, basis sets, solvation models and thermostatical analysis tools to achieve modeling accuracy and robustness.
c) Accessing novel sp3 rich glycosidic chemotypes and deconvoluting novel biological activity
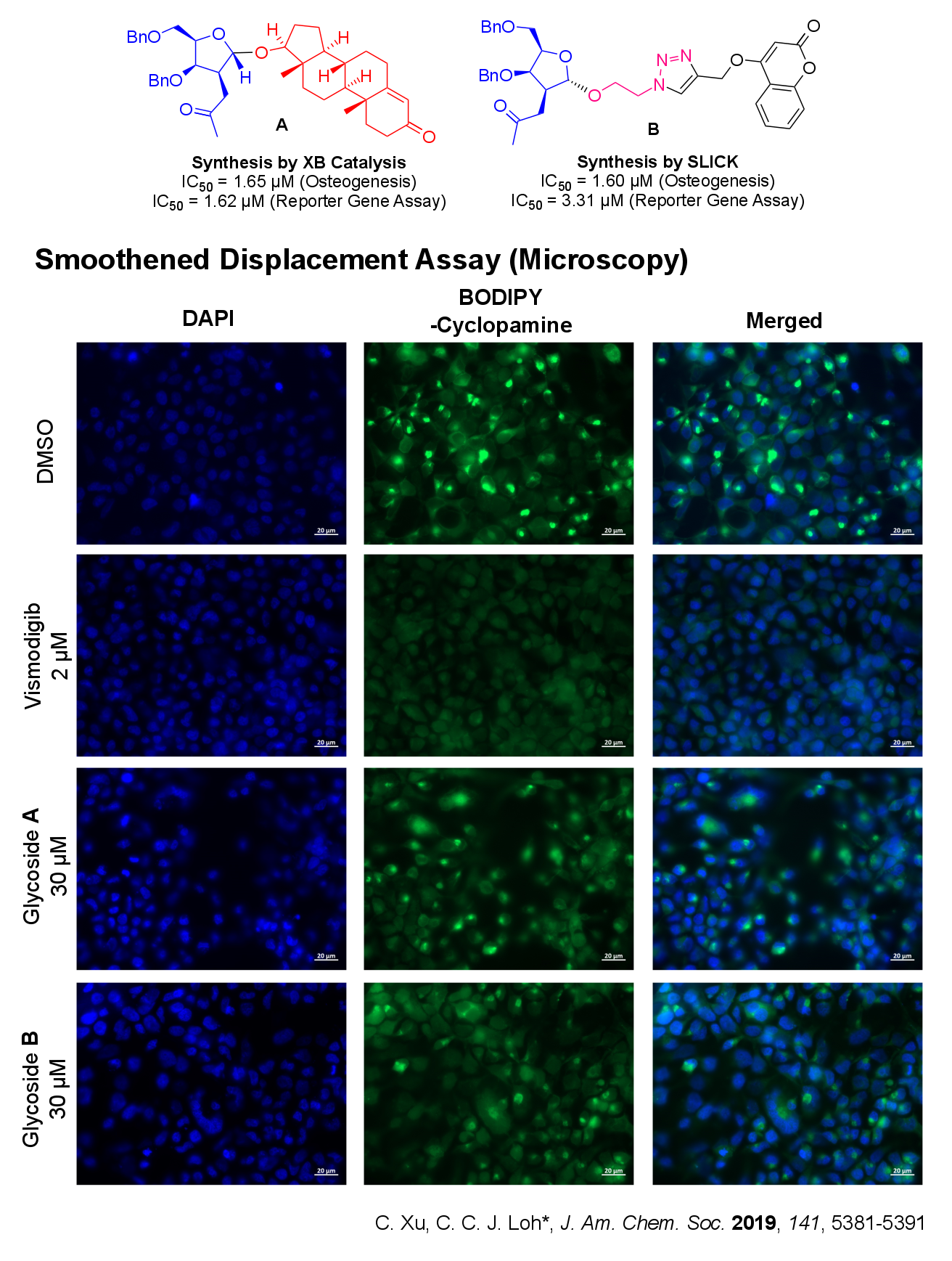
We apply our expertise in synthesizing unnatural glycosides and bridge it to real life biological applications via a forward chemical genetics approach (from phentoype to gene), i.e. exploit phenotypic screens as a powerful interrogation tool to uncover new glycosidic analogues that have potential applications in oncology (cancer). We are particularly interested in developmental pathways that involve cross-talk of different biological mechanisms (e.g. in osteogenesis).
Accessing non-natural carbohydrates through our developed methods is of particular interest as they are endowed with unique sp3 rich 3-dimensionality unseen in other more common chemotypes, which are mostly flat and sp2 rich.
Currently our analogues derived from XB catalyzed strain release glycosylation, and our SLICK methodology has proven effective in inhibiting the Hedgehog (Hh) Signaling Pathway. More significantly, using chemical biological techniques such as cell based assays, fluorescence activated cell sorting (FACS), fluorescence and confocal microscopy, we established that our strain-release glycosides define a new class of non-Smoothened Hh inhibitors that has potential applications against acquired cancer resistance.