Publications List
Independent Career
31) H. Guo, C. C. J. Loh* Sci. Bull. 2024, DOI: 10.1016/j.scib.2024.08.035 (invited contribution to highlight Prof. Dawen Niu's work)
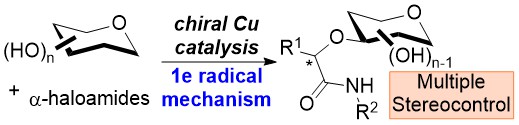
30) H. Guo, D. Tan, C. Merten, C. C. J. Loh* Angew. Chem. Int. Ed. 2024, DOI: 10.1002/anie202409530
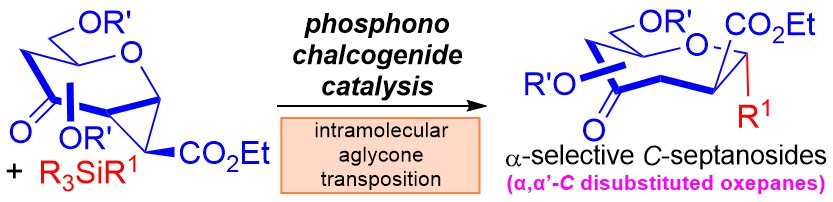
29) W. Ma, A. Schmidt, C. Strohmann, C. C. J. Loh* Angew. Chem. Int. Ed. 2024, 63, e202405706
(Selected as Hot Paper in Angew. Chem.)
28) C. C. J. Loh* Chem. Catal. 2024, 4, 100980. (invited contribution to highlight Prof. Gang He's work in glycosylidene carbenes)
27) C. Wang, A. Krupp, C. Strohmann, B. Grabe, C. C. J. Loh* J. Am. Chem. Soc. 2024, 146, 10608-10620.
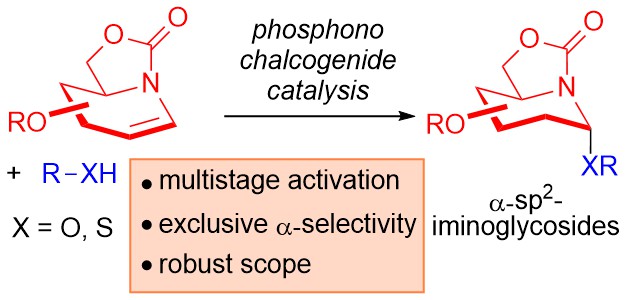
26) H. Guo, J-L. Kirchhoff, C. Strohmann, B. Grabe, C. C. J. Loh* Angew. Chem. Int. Ed. 2024, 63, e202400912.
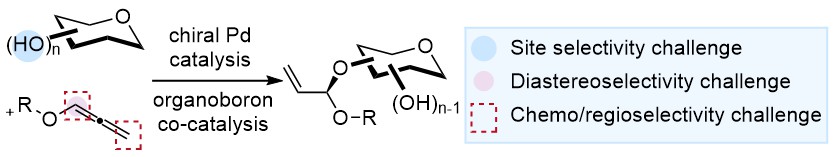
25) C. C. J. Loh* Chem. Catal. 2024, 4, 100891. (invited)
Synergistic catalysis: An emerging concept for selective carbohydrate synthesis.
In this perspective, Charles discuss his views pertaining to the renaissance of the synthetic-discovery-centered paradigm in stereoselective carbohydrate synthesis through emerging catalytic concepts such as synergistic catalysis.
![]()
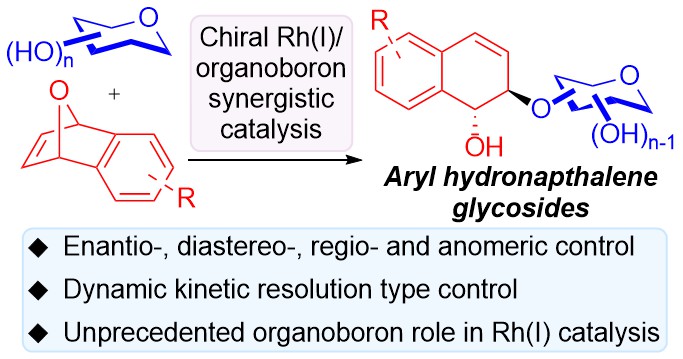
24) H. Guo, J-L. Kirchhoff, C. Strohmann, B. Grabe, C. C. J. Loh* Angew. Chem. Int. Ed. 2024, 63, e202316667.
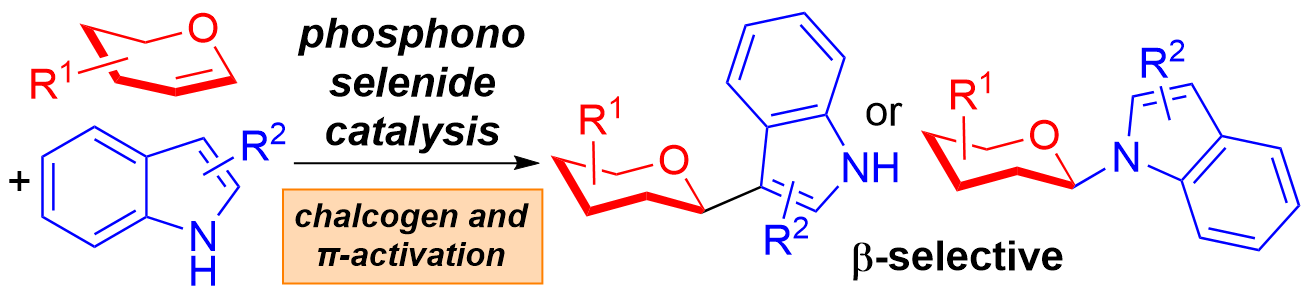
23) W. Ma, J-L. Kirchhoff, C. Strohmann, B. Grabe, C. C. J. Loh* J. Am. Chem. Soc. 2023, 145, 26611-26622.
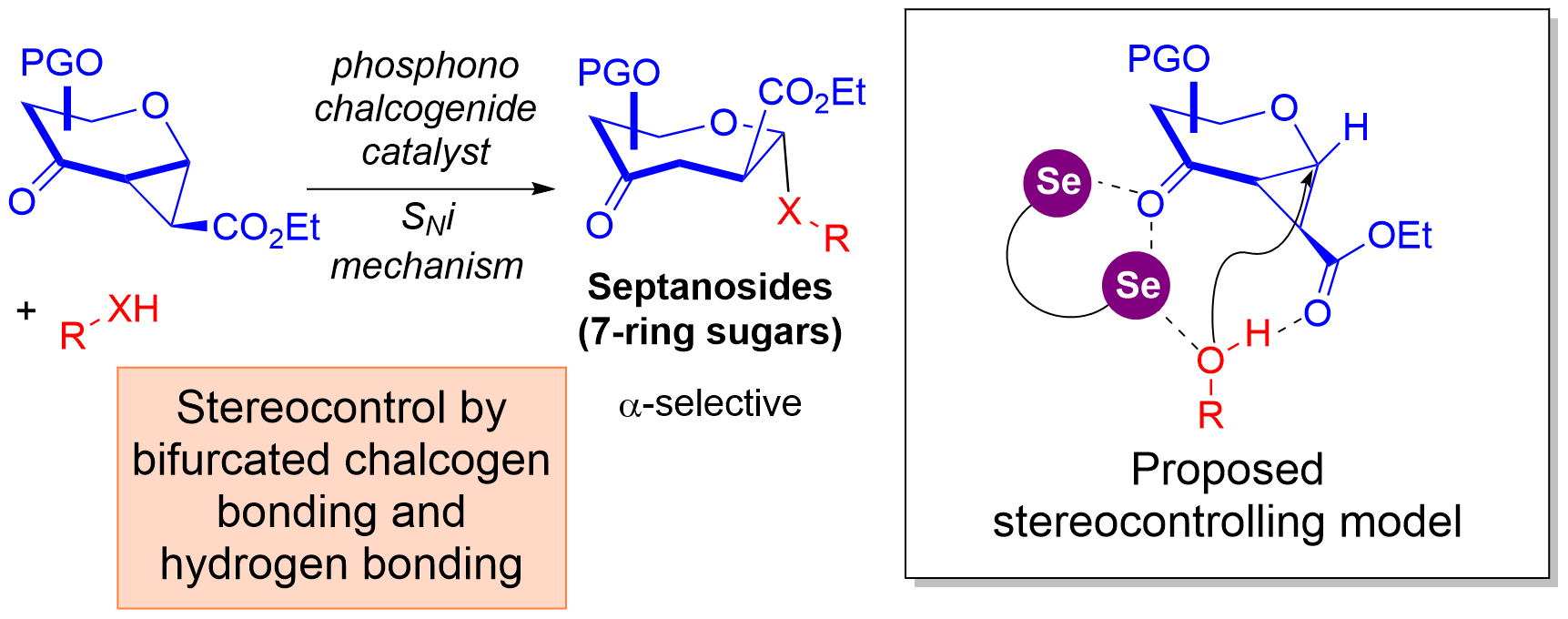
22) V. U. Bhaskara Rao,† C. Wang,† D. P. Demarque, C. Grassin, F. Otte, C. Merten, C. Strohmann, C. C. J. Loh* Nat. Chem. 2023, 15, 424-435.
†Authors contributed equally
Highlighted by X-MOL (link)
Highlighted by "化学加" (link)
21) C. C. J. Loh*, Nat. Rev. Chem. 2021, 5, 792-815.
Exploiting Non-Covalent Interactions in Selective Carbohydrate Synthesis.
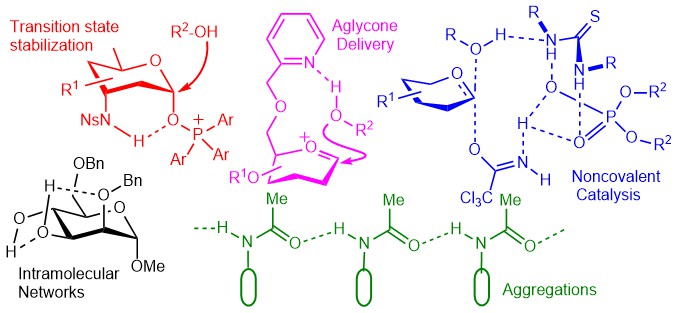
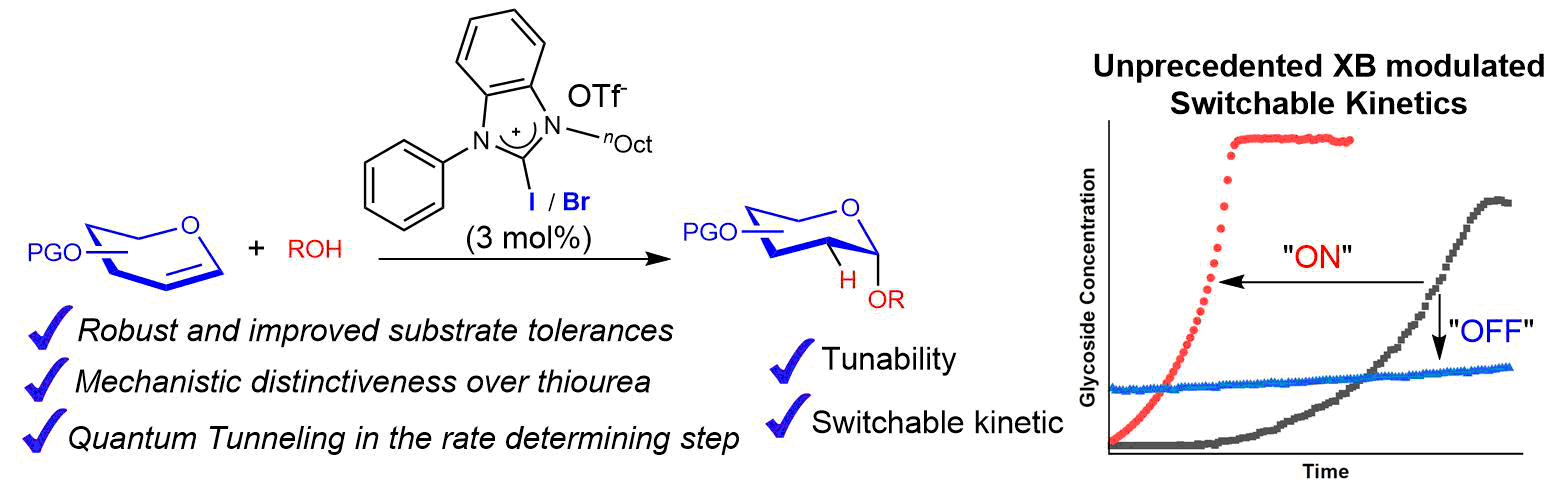
20) C. Xu, V. U. Bhaskara Rao, J. Weigen, C. C. J. Loh*, Nat. Commun. 2020, 11, 4911.
A Robust and Tunable Halogen Bond Organocatalyzed 2-Deoxyglycosylation Involving Quantum Tunneling.
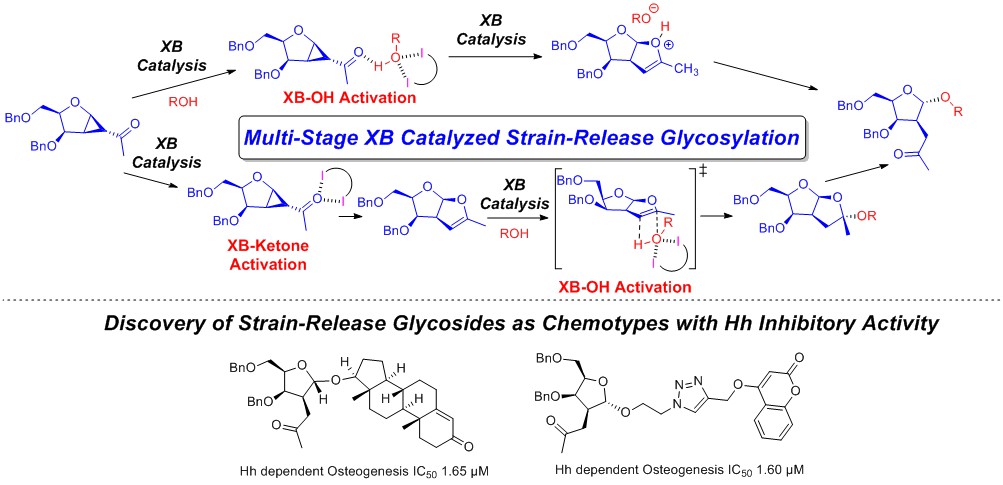
19) C. Xu, C. C. J. Loh*, J. Am. Chem. Soc. 2019, 141, 5381-5391.
A Multi-stage Halogen Bond Catalyzed Strain-Release Glycosylation unravels New Hedgehog Signaling Inhibitors.
- Highlighted in Synfacts 2019, 15, 0666, Contributors B. List, M. Turberg
- Highlighted in Nachrichten aus der Chemie 2020, 68, 42-72.
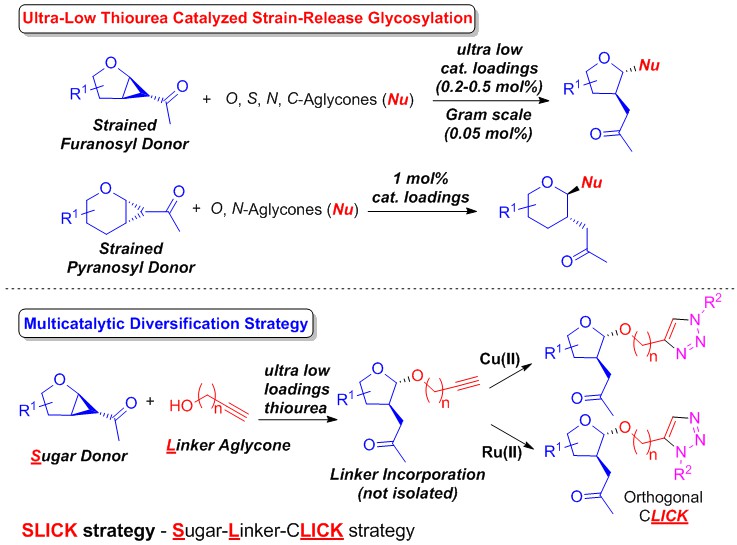
18) C. Xu, C. C. J. Loh*, Nat. Commun. 2018, 9, 4057.
An ultra-low thiourea catalyzed strain-release glycosylation and a multicatalytic diversification strategy.
Before Independent Career (RWTH Aachen and University of Toronto)
17) A. Yen, K-L. Choo, S. K. Yazdi, P. T. Franke, R. Webster, I. Franzoni, C. C. J. Loh, A. I. Poblador-Bahamonde, M. Lautens*, Angew. Chem. Int. Ed. 2017, 56, 6307-6311
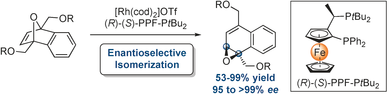
16) C. C. J. Loh, M. Schmid, R. Webster, A. Yen, S. K. Yazdi, P. T. Franke, M. Lautens*, Angew. Chem. Int. Ed. 2016, 55, 10074-10078.
Rhodium Catalyzed Asymmetric Cycloisomerization and A Parallel Kinetic Resolution of Racemic Oxabicycles.
(Highlighted in Synfacts 2017, 13, 0174, Contributors H. Yamamoto, H. Tsuji)
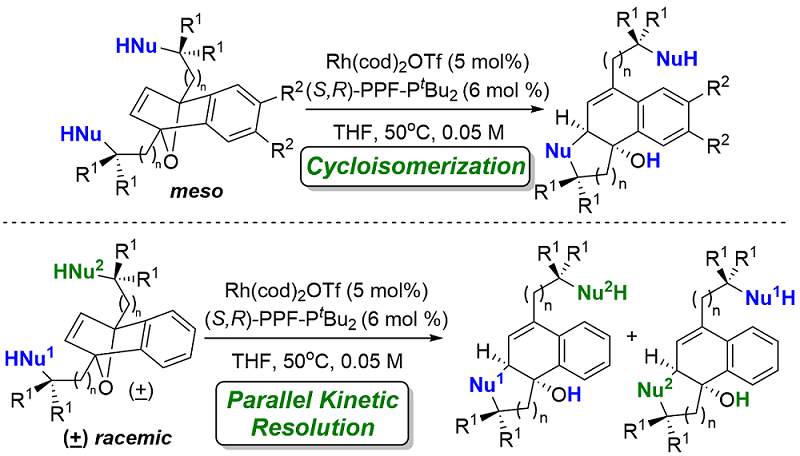
15) C. C. J. Loh, M. Schmid, B. Peters, X. Fang, M. Lautens*, Angew. Chem. Int. Ed. 2016, 55, 4600-4604.
Exploiting Distal Reactivity of Coumarins: A Rhodium Catalyzed Vinylogous Asymmetric Ring Opening Reaction.
(Highlighted in Synfacts 2016, 12, 0719, Contributors H. Yamamto, F. Zhou)
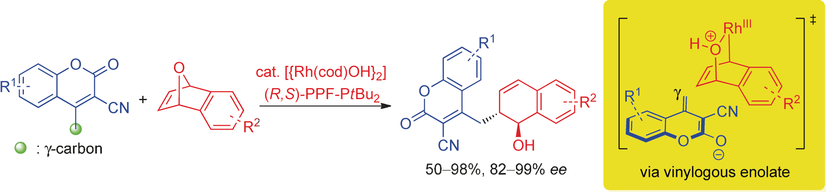
14) C. C. J. Loh, X. Fang, B. Peters, M. Lautens*, Chem. Eur. J. 2015, 21, 13883-13887.
Benzylic Functionalization of Anthrones via the Asymmetric Ring Opening of Oxabicycles utilizing a Fourth Generational Rhodium Catalytic System.
(Highlighted in Synfacts 2015, 11, 1286, Contributors H. Yamamoto, F. Zhou)
13) S. Mahajan, P. Chauhan, C. C. J. Loh, S. Uzungelis, G. Raabe, D. Enders*, Synthesis 2015, 47, 1024-1031.
Organocatalytic Asymmetric Domino Michael/Henry Reaction of Indolin-3-ones with o-Formyl-b-nitrostyrenes.
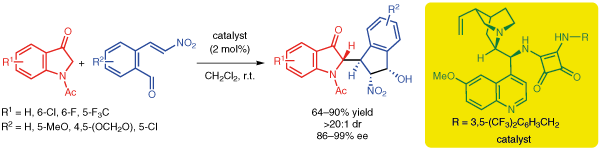
12) P. Chauhan, S. Mahajan, C. C. J. Loh, G. Raabe, D. Enders*, Org. Lett. 2014, 16, 2954-2957.
Stereocontrolled Construction of Six Vicinal Stereogenic Centers on Spiropyrazolones via Organocascade Michael/Michael/1,2-Addition Reactions.
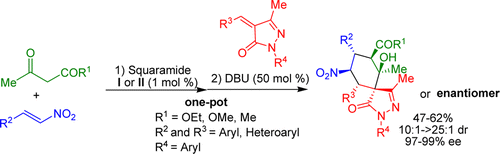
11) D. Hack, C. C. J. Loh, J. M. Hartmann, G. Raabe, D. Enders*, Chem. Eur. J. 2014, 20, 3917-3921.
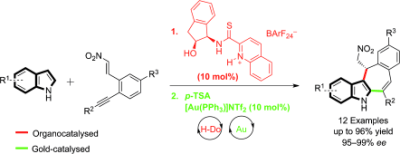
Merging Gold and Organocatalysis: A Facile Asymmetric Synthesis of Annulated Pyrroles.
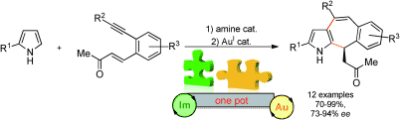
10) C. C. J. Loh, P. Chauhan, D. Hack, C. Lehmann, D. Enders*, Adv. Synth. Catal. 2014, 356, 3181-3186.
Rapid Asymmetric Synthesis of Highly Functionalized Indanols via a Michael/Henry Organocascade with Submol% Squaramide Catalyst Loadings.
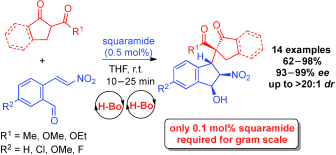
9) Q. Ni, H. Zhang, A. Grossmann, C. C. J. Loh, C. Merkens, D. Enders*, Angew. Chem. Int. Ed. 2013, 52, 13562-13566.
Asymmetric Synthesis of Pyrroloindolones via N-Heterocyclic Carbene Catalyzed [2+3]-Annulation of α-Chloroaldehydes with Nitrovinylindoles.
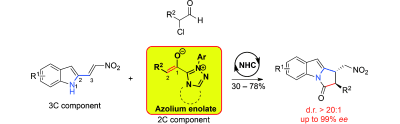
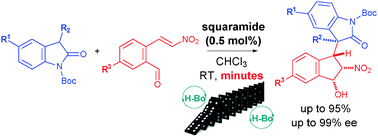
8) C. C. J. Loh, D. Hack, D. Enders*, Chem. Commun. 2013, 49, 10230-10232
Asymmetric Domino Synthesis of Indanes bearing Four Contiguous Stereocentres catalyzed by Sub-mol% Loadings of a Squaramide in Minutes.
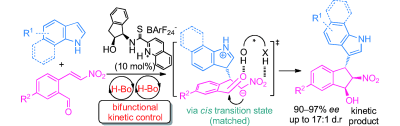
7) C. C. J. Loh, I. Atodiresei, D. Enders*, Chem. Eur. J. 2013, 19, 10822-10826.
Asymmetric Organocatalytic Michael/ Henry Domino Reactions through Hydrogen Bond Activation: A Kinetic Access into Indane Scaffolds Bearing cis-vicinal Substituents.
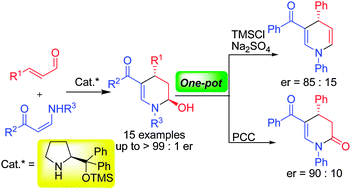
6) J.-P. Wan, C. C. J. Loh, F. Pan, D. Enders*, Chem. Commun. 2012, 48, 10049-10051.
Enantioselective Organocatalytic Domino Synthesis of Tetrahydropyridin-2-ols.
5) C. Wang, X. Yang, C. C. J. Loh, D. Enders*, Chem. Eur. J. 2012, 18, 11531-11535.
Organocatalytic, Asymmetric Synthesis of 3-Sulfenylated N-Boc-Protected Oxindoles.
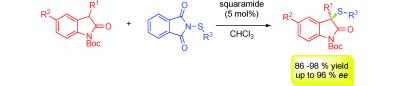
4) C. C. J. Loh, G. Raabe, D. Enders*, Chem. Eur. J. 2012, 18, 13250-13254.
Enantioselective Synthesis of Tetrahydrocarbazoles Through a Michael Addition/ Ciamician-Plancher Rearrangement Sequence. Asymmetric Synthesis of a Potent Constrained Analog of MS-245.
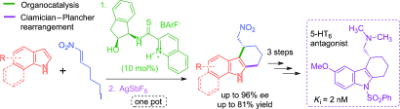
3) C. C. J. Loh, D. Enders*, Chem. Eur. J. 2012, 18, 10212-10225.
Merging Organocatalysis and Gold Catalysis—A Critical Evaluation of the Underlying Concepts. (Minireview) (Special conference issue for 4th Chemistry European Congress, invited)
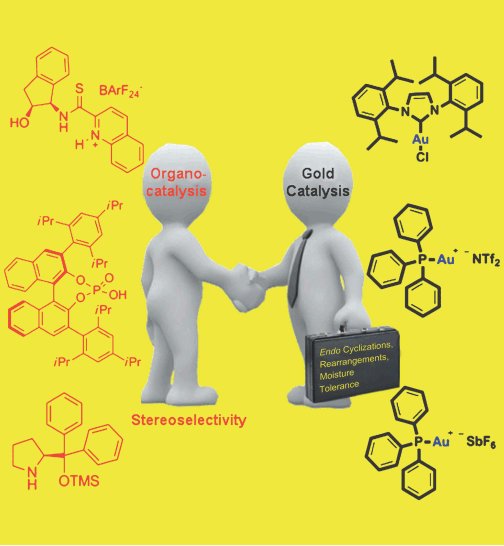
2) C. C. J. Loh, D. Enders*, Angew. Chem. Int. Ed. 2012, 51, 46-48.
Exploiting the Electrophilic Properties of Indole Intermediates: New Options in Designing Asymmetric Reactions.
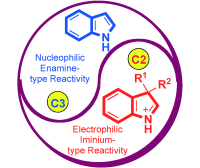
1) C. C. J. Loh, J. Badorrek, G. Raabe, D. Enders*, Chem. Eur. J. 2011, 17, 13409-13414.
Merging Organocatalysis and Gold Catalysis: Enantioselective Synthesis of Tetracyclic Indole Derivatives through a Sequential Double Friedel–Crafts Type Reaction.
(Highlighted in Synfacts 2012, 8(1), 97, Contributors B. List, M. Mahlau).