The research work in the Loh research group harnesses nature inspired noncovalent interactions (NCIs) and asymmetric catalysis concepts to advance carbohydrate chemistry and general biomolecule synthesis.
We use a mechanistic-driven approach to understand NCI intricacies of catalytic glycosylation/glycofunctionalization pathways, in particular by kinetic experiments and DFT computations. We have a long-term interest in unraveling synthetic capabilities of the broad palette of NCIs including and beyond the ubiquitous hydrogen bond.
Our research programme is uniquely interdisciplinary, spanning a range of chemistry fields including synthesis, methodology development, catalysis, supramolecular chemistry, computational chemistry and chemical biology. This modern intersectional approach opens up a plethora of opportunities to answer innovative research questions at the interface of major chemical research themes. Our research philosophy is firmly grounded on innovation and discovery of chemical phenomena in the broadest sense, and not restricted by conventional boundaries.
Conceptually, we build bridges between the general synthetic/catalysis and the carbohydrate community through investigating carbohydrates as an excellent platform for catalytic method development. We also discover new catalytic σ-hole phenomena and concepts that is of interest to the noncovalent interaction/coordination chemistry community. We are excited to contribute through the rapidly emerging modern paradigm that recognizes carbohydrate synthesis as an excellent incubator to advance fundamental synthetic knowledge. Link
Our group conceptualized the harnessing of ring strain as a robust glycosylation concept, a named-strategy that we first coined and now widely recognized in the community as "strain-release glycosylation" - A versatile transformation on the sugar's anomeric C1 that involves ring-strain release within a glycosylation mechanistic manifold. This promising concept and it's terminology is now expanding and rapidly assimilated by other research groups.
Our research direction is currently divided into the following lines:
a) Establishment of the σ-hole based noncovalent catalytic strategy in stereoselective carbohydrate synthesis
Inspired by how enzymes such as glycosyl-transferases perform precision stereocontrolled chemistry , our central concept involved investigating how non-classical σ-hole based noncovalent interactions such as halogen bonding (XB) and chalcogen bonding (ChB) could tackle long-standing selectivity issues in carbohydrate functionalization.
A detailed narration of our discovery journey is now found in Acc. Chem. Res. 2025, 58, 2124-2144 (Link)
Retrospectively, it is worthwhile to mention that prior to 2019, there was no indication in the literature of the transformative role that σ-hole based NCIs we see today in the carbohydrate chemistry arena.
First instances of exclusively XB catalyzed glycosylations (2018-2020)
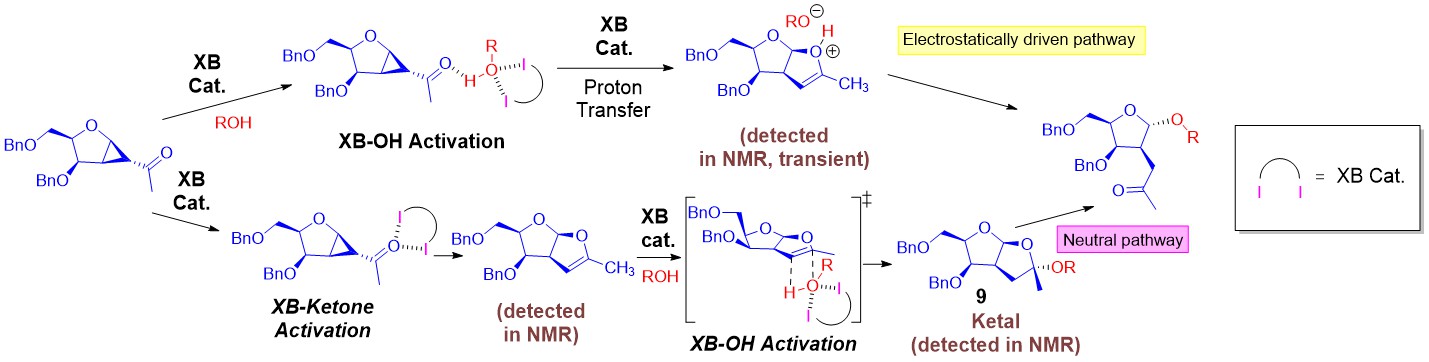
In 2019, our group reported our seminal work in the discovery of XB catalysis to construct glycosidic bonds via versatile strain-release glycosylations. Two defining discoveries were made:
(1) The unraveling and experiment proof of reversible iterative σ-hole···O interactions in catalysis.
(2) The amenability of multiple σ-hole based activation on glycosyl substrates, intermediates along the entire reaction coordinate in complex mechanistic settings.
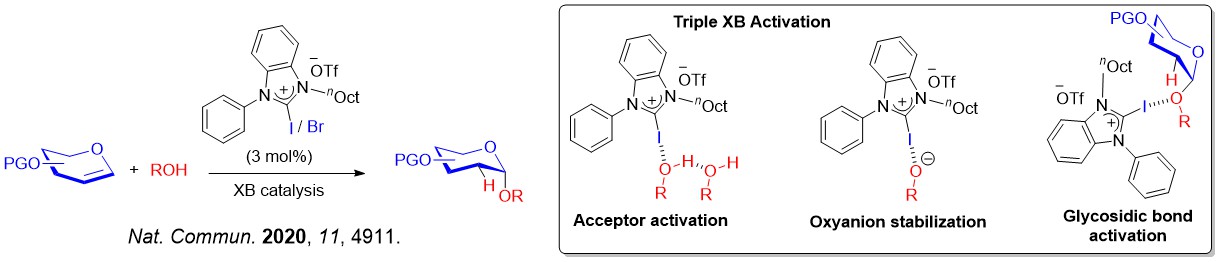
The XB catalyzed glycosylation strategy that involved iterative σ-hole···O interactions was expanded in other glycosylation platforms, such as 2-deoxyglycosylations that showed clear robustness over classical Schreiner's thiourea catalyzed methods.
Debut of ChB catalyzed glycosylations (2023 onwards)
We advanced the field by introducing the robust chalcogen bonding catalyzed glycosylation in 2023. Benefiting from the 2 σ-hole per chalcogen atom on phosphonochalcogenide (PCH) catalysis, we discovered a robust O- and S-septanosylation strategy that involves a SNi mechanism.
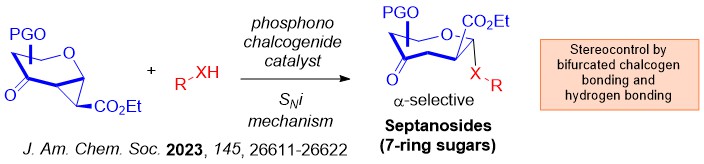
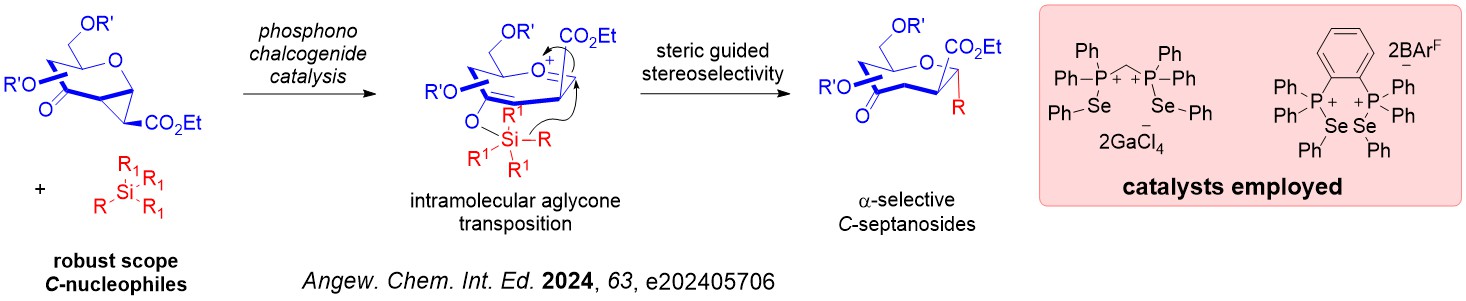
Interesting, a mechanistic shift to a steric guided intramolecular aglycone transposition pathway was discovered using silylated C-nucleophiles.
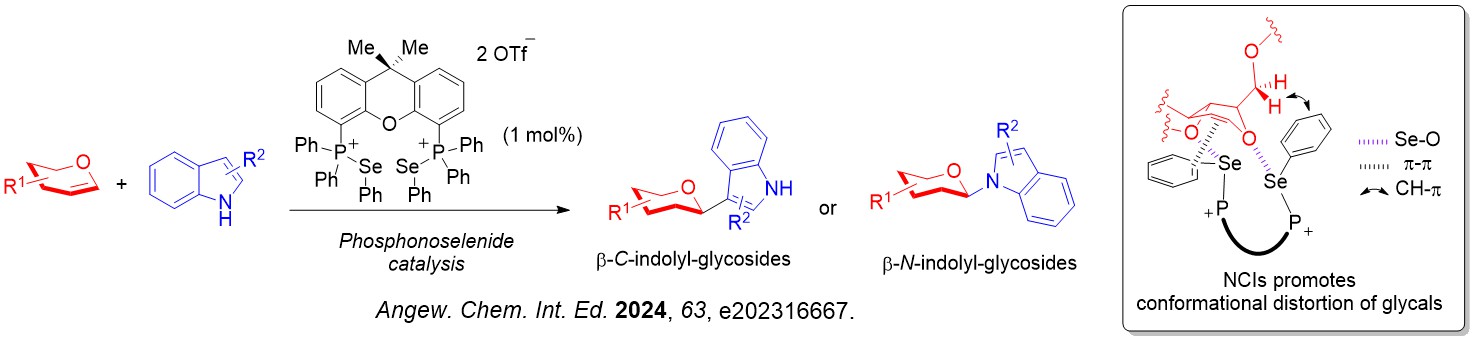
We also discovered that biologically important β-indolyl glycosides can be accessed through an unprecedented glycosyl donor conformation distortion strategy.
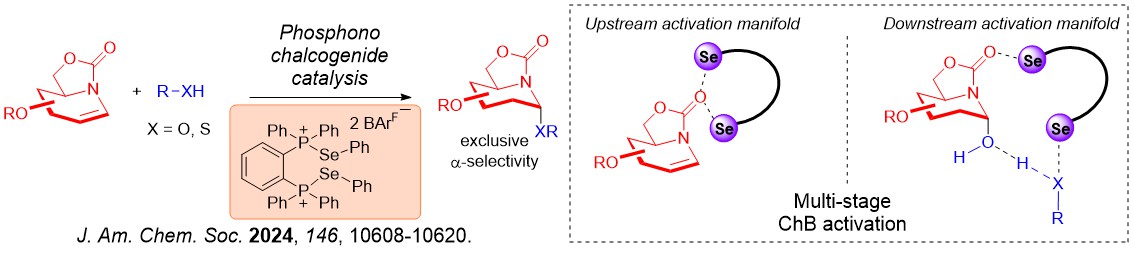
Furthermore, glycomimetics such as iminoglycosides can also be robustly accessed using ChB catalysis. We further discovered that the iterative σ-hole···O interactions we first observed on XB catalysis is also operative using ChB catalysis.
b) Advancing asymmetric catalysis in the realm of site-selective carbohydrate functionalizations.
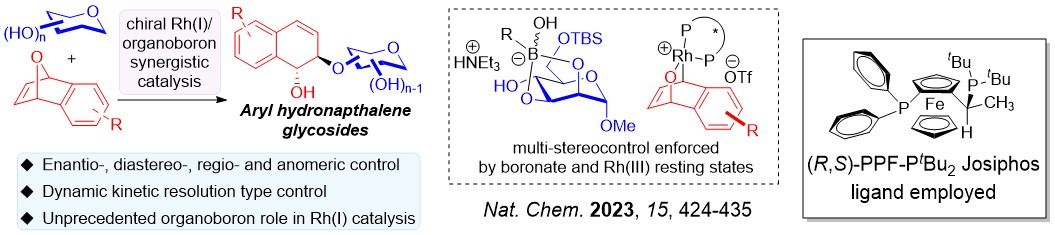
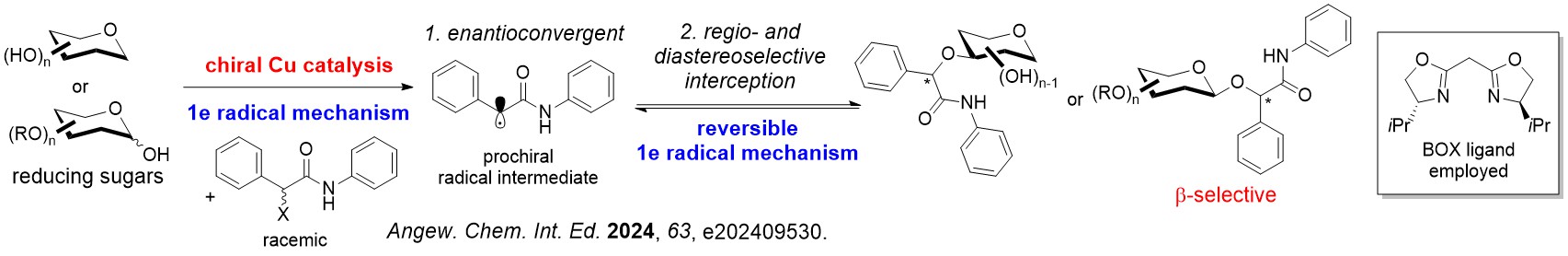
We have discovered several asymmetric catalytic systems, spanning from rhodium, palladium and copper radical catalysis to concomittantly address multiple stereoselectivity challenges with a single C-O bond forming step in carbohydrate polyols.
Differing from classical asymmetric catalysis, assimilating chiral catalysis on carbohydrate polyols uniquely involves tackling two difficult stereoselectivity challenges: (i) Site/Regioselectivity; (ii) Anomeric Selectivity.
Other important features of stereoselectivity control such as chiral catalyst-controlled dynamic kinetic resolution type glycosylations are also advanced by our group.

c) Mechanistic Investigations via synergy of experimental physical organic chemistry and computations
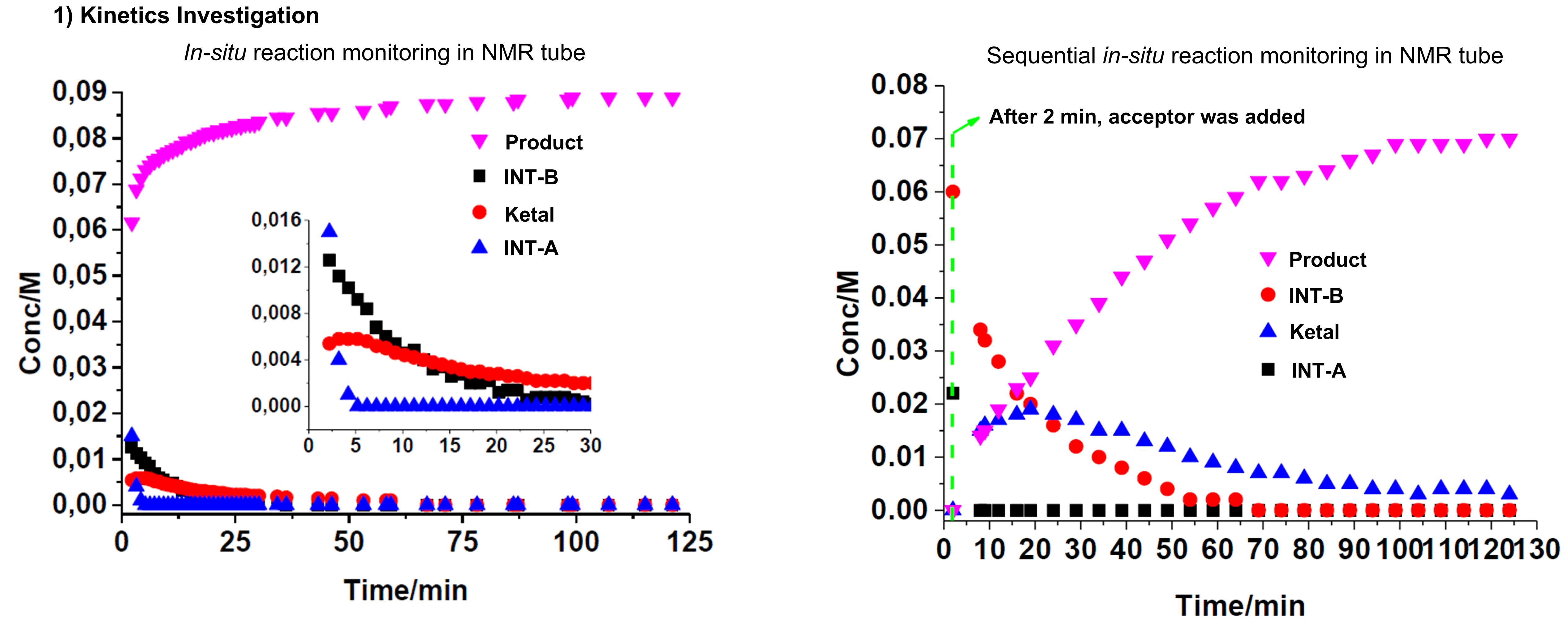
We are interested to advance carbohydrate chemistry by unraveling the mechanistic intricacies via rigorous in-situ NMR and IR-monitoring, linear free energy relationships and kinetic analysis (e.g. reaction orders). This physical organic chemistry grounded, mechanistic-driven approach is a powerful tool that enables us to dissect the glycosylation pathways involving NCIs.
We take an active questioning approach in every single new method we develop to probe the mechanism without pre-supposition. We have shown in multiple examples that new mechanistic pathways are always out there to be discovered. We are driven to discover new mechanisms through an open-minded philosophy to surprises, be curiosity-driven, to let our conclusions only follow the data observed.
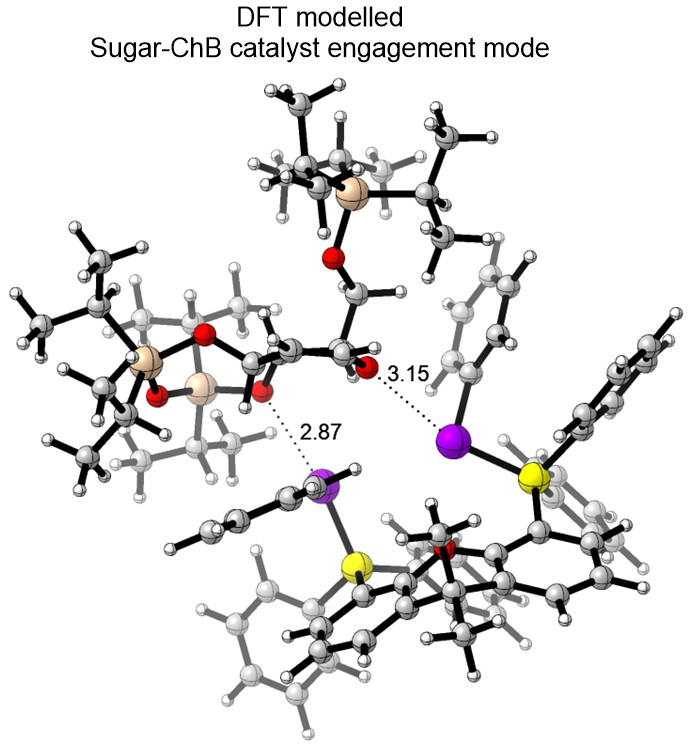
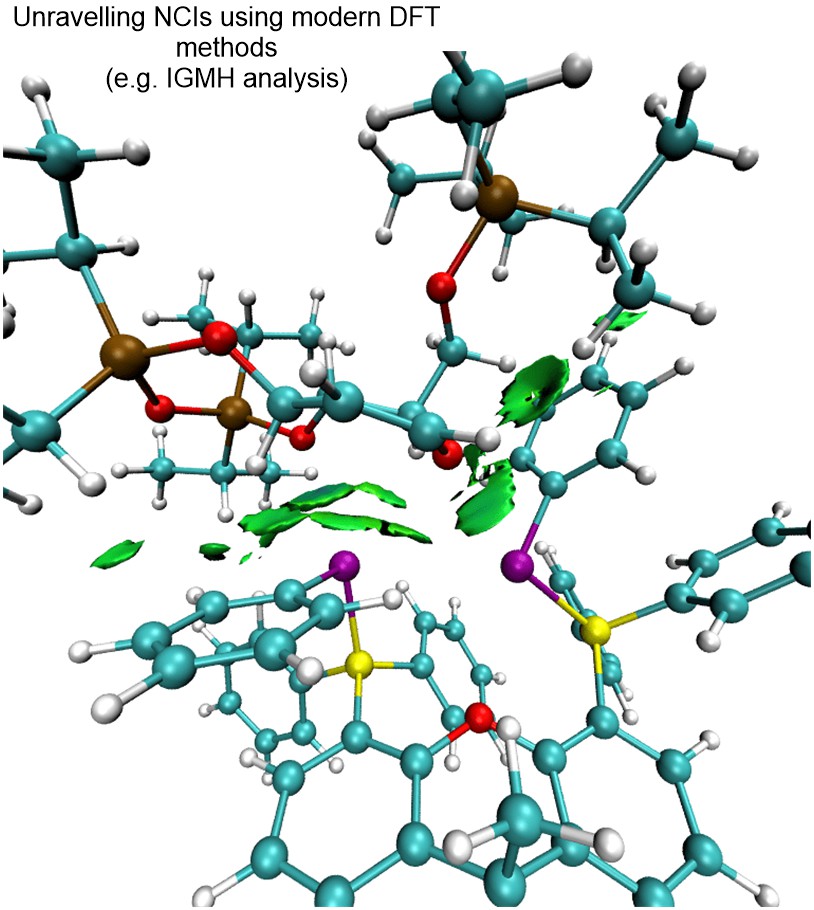
We have currently also established an in-house platform for modern DFT calculations, and we harness a range of frontier computational tools to provide theoretical support for mechanistic questions that involves NCIs. We employ state-of-the-art DFT functionals, basis sets, solvation models and thermostatical analysis tools to achieve modeling accuracy and robustness.
d) Accessing novel sp3 rich glycosidic chemotypes and deconvoluting novel biological activity
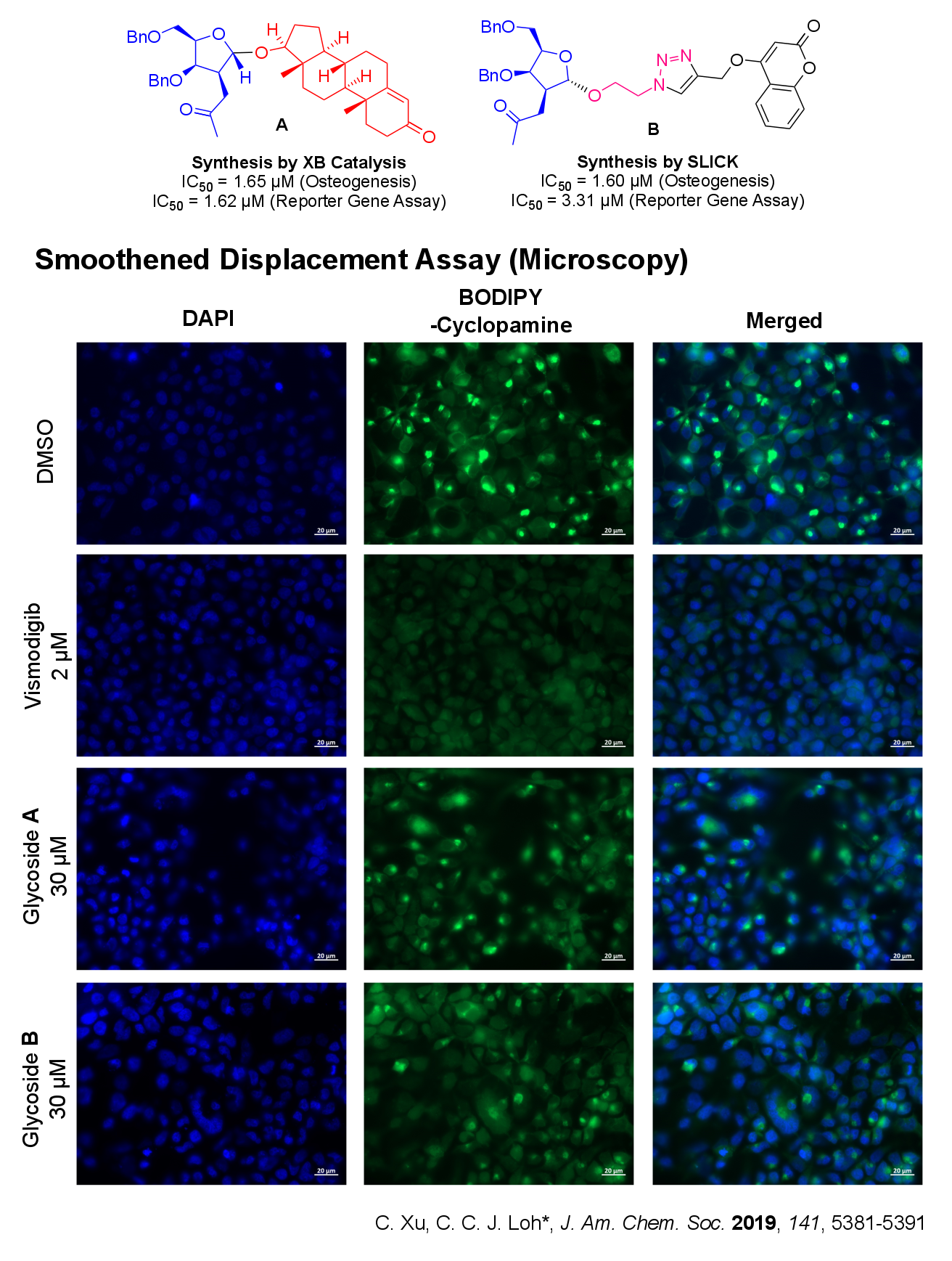
We apply our expertise in synthesizing unnatural glycosides and bridge it to real life biological applications via a forward chemical genetics approach (from phentoype to gene), i.e. exploit phenotypic screens as a powerful interrogation tool to uncover new glycosidic analogues that have potential applications in oncology (cancer). We are particularly interested in developmental pathways that involve cross-talk of different biological mechanisms (e.g. in osteogenesis).
Accessing non-natural carbohydrates through our developed methods is of particular interest as they are endowed with unique sp3 rich 3-dimensionality unseen in other more common chemotypes, which are mostly flat and sp2 rich.
Currently our analogues derived from XB catalyzed strain release glycosylation has proven effective in inhibiting the Hedgehog (Hh) Signaling Pathway. More significantly, using chemical biological techniques such as cell based assays, fluorescence activated cell sorting (FACS), fluorescence and confocal microscopy, we established that our strain-release glycosides define a new class of non-Smoothened Hh inhibitors that has potential applications against acquired cancer resistance.